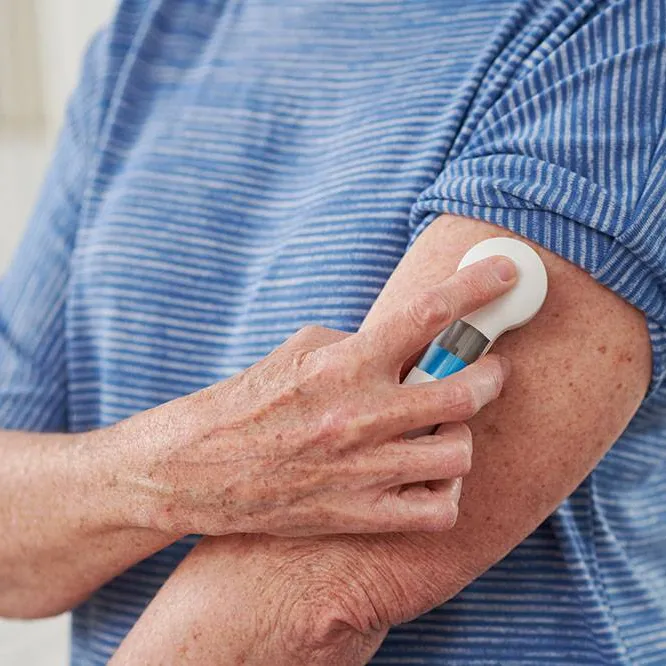
Microstructured Transdermal Systems (MTS)
Drug Delivery
Information
Kindeva’s MTS platform provides elegant microneedle-based delivery devices, refined and suited to our partners’ programs.
Analytical & technical leadership:
From early-phase feasibility to final design requirements, we can meet the needs of challenging drug formulations.
• Feasibility and early-phase development capabilities
• Human tolerability and human factors studies
• Proven ability to develop challenging drug formulations
• Preclinical and clinical capabilities
• Regulatory leadership and expert data analysis to support submissions and filings
Innovative devices:
Refined over several device generations, Kindeva’s microstructured systems are designed for consistent intradermal delivery of vaccines and biologics. Our patient-friendly technology is available in two formats, providing an option of delivery routes to enable your commercial success.
• Solid Microneedles – offers potential for increased immunogenicity, dose sparing, cold chain elimination, and increased patient convenience via self-administration
• Hollow Microneedles – offers reproducible intradermal delivery of a wide range of viscosities
Operational excellence:
With sophisticated project management, rigorous quality processes, manufacturing excellence, and cGMP facilities, Kindeva supports a reliable supply chain for your program.
• Automated Array Molding and Packaging
• Aseptic Array Coating for Solid Microneedles
• Qualified Equipment for Device Assembly Process
• Manufacturing capability for Preclinical, Clinical, and Scale-up Activities
