Aegyris™ The Simple and Efficient Solution for the Validation of Immunogenicity Assays to Support Vaccine Clinical Trials
Poster Zone Area
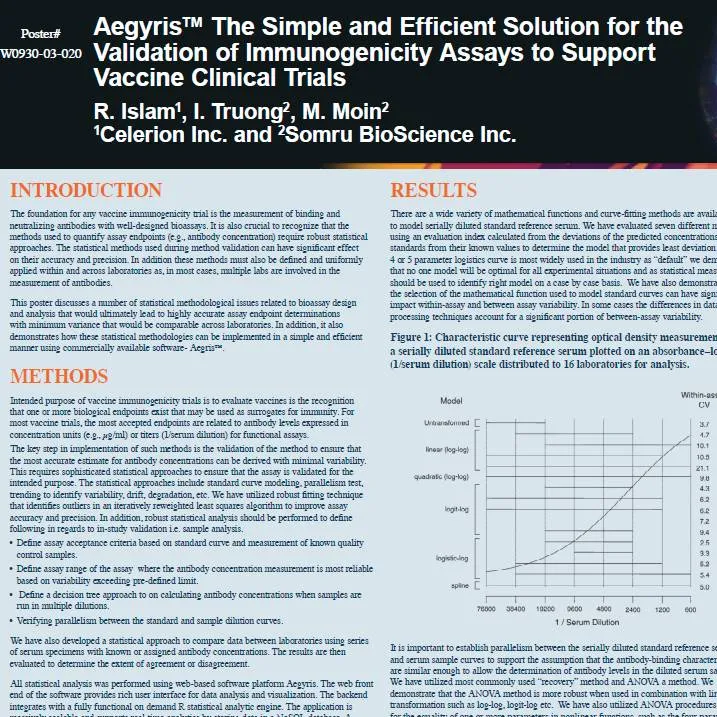
Information
The foundation for any vaccine immunogenicity trial is the measurement of binding and neutralizing antibodies with well-designed bioassays. It is also crucial to recognize that the methods used to quantify assay endpoints (e.g., antibody concentration) require robust statistical approaches. The statistical methods used during method validation can have significant effect on their accuracy and precision. In addition these methods must also be defined and uniformly applied within and across laboratories as, in most cases, multiple labs are involved in the measurement of antibodies. This poster discusses a number of statistical methodological issues related to bioassay design and analysis that would ultimately lead to highly accurate assay endpoint determinations with minimum variance that would be comparable across laboratories. In addition, it also demonstrates how these statistical methodologies can be implemented in a simple and efficient manner using commercially available software- Aegris™.
